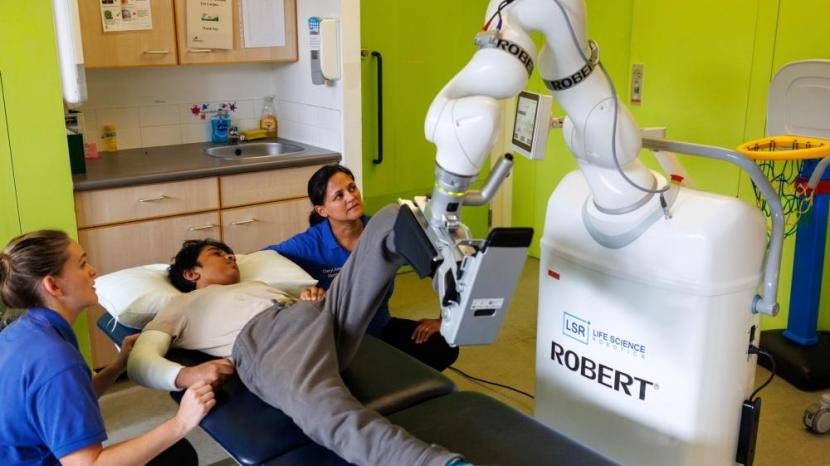
First UK paediatric ROBERT® trial commences at The Children’s Trust
The first paediatric trial of the ROBERT® in the UK has commenced with The Children’s Trust.
The Children’s Trust will be using the ROBERT®, a robotic rehabilitation device for early upper and lower limb mobilisation, for the next three months with children and young people placed with them for specialist neurorehabilitation.
ROBERT® is developed by Life Science Robotics and represented in the UK by Summit Medical and Scientific.
We are delighted to be working alongside Summit Medical and Scientific as the only paediatric provider in the UK selected as part of the ROBERT ® trial. The device offers intensive mobilisation of a patient’s limbs and helps to get them moving as soon as possible which is vital in optimising recovery and facilitating neuroplasticity. We will be integrating ROBERT ® into our neurorehabilitation programmes where clinically appropriate and we are excited to evaluate the impact on outcomes in due course, Melanie Burrough, Director of Therapies and Education at The Children’s Trust said."
About the ROBERT®
ROBERT® is a robotic rehabilitation device, offering a complete and flexible solution for upper and lower limb therapy.
ROBERT® provides intensive rehabilitation for acute patients as early as possible, and can be used for upper or lower limb therapy either in-bed or seated with just one device.
We are delighted to support The Children’s Trust and their valuable work with young patients in the UK. Access to robotic rehabilitation can support the early, high quality and intensive therapy that makes a vital difference to a child’s recovery, confidence and quality of life. We are looking forward to hearing from these inspiring patients and therapists about their experiences of using the ROBERT®, Sara Brammall, Managing Director at Summit Medical and Scientific, said.“
Even severely impaired patients can be mobilised using ROBERT®, supporting a patient’s rehabilitation as soon as possible after their admission to hospital. Getting patients moving early and intensively is an important step towards optimal recovery and facilitating neuroplasticity.
Therapists first define the desired movement using ROBERT®, including Activities of Daily Living and Proprioceptive Neuromuscular Facilitation with seven degrees of freedom.
We are proud to see ROBERT® being utilized for the first time into a paediatric setting, at The Children’s Trust. Helping children regain mobility and improve their quality of life is at the core of our mission, and we are thrilled to be contributing to their recovery journey. We look forward to seeing the positive impact ROBERT® can have on young patients’ rehabilitation and their progress, Keld Thorsen, CEO at Life Science Robotics, said."
The ROBERT® then repeats the intensive therapy, offering tailored levels of resistance and support based on the patient’s ability. Passive mobilisation is for completely immobilised patients, where ROBERT® carries out the exercise on the behalf of patients with reduced or no function. Active assistive mobilisation guides the patient through the exercise, and the patient’s function and strength determine the level of activity. Finally, active resistive mobilisation offers resistance against the patient’s movement.
ROBERT®’s assistive technology reduces the need for heavy and repetitive lifts, freeing resources for other aspects of clinical treatment and reducing the strain on the therapist. Clinicians can transport the device throughout their facility to each patient, and simply plug, attach, record and play using the intuitive interface. Patients can be set up and ready to go in as little as three minutes.
For press and media information please contact:
Summit Medical and Scientific on 01372 459863 or email info@summitmedsci.co.uk
The Children’s Trust Press Office on 01737 364 325 or email pressoffice@thechildrenstrust.org.uk